A Star Produces a Continuous Spectrum From Its
PHYSICS 1040 - ELEMENTARY ASTRONOMY - HOMEWORK #3
1. When white sunlight passes through a prism, it is broken into a rainbow-like continuous spectrum. Joseph Fraunhofer discovered that the Sun's spectrum contains many dark spectral lines. Each atom or molecule has its own pattern of characteristic spectral lines. By studying these lines, astronomers can determine which atoms and molecules are found on the Sun, planets, and other stars.
2. A hot dense object or gas gives off blackbody radiation. This consists of light of all colors (wavelengths), so when this light passes through a prism it produces a continuous spectrum. Because a star is a ball of hot dense gas, it behaves like a blackbody.
3. Suppose the temperature (in kelvins) of blackbody 1 is three times as great as that of blackbody 2. Then the energy released per second by each square meter of the surface of blackbody 1 is 3x3x3x3=81 times the energy released per second by each square meter of the surface of blackbody 2.
4. Wien's Law says that the peak wavelength of a blackbody spectrum is
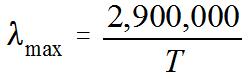
in units on nanometers (nm). (1 nm = 1 x 10-9 m.) So, if you are given the temperature T (in kelvins) of an object, you can find the wavelength at which most of the light is emitted.
a. For gas spiraling into a black hole, T = 1,000,000 K, so
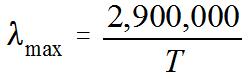 = 2.9 nm.
= 2.9 nm.
This is in the x-ray part of the electromagnetic spectrum.
b. For a star with surface temperature T = 25,000 K, so
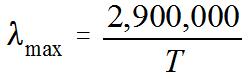 = 116 nm.
= 116 nm.
This is in the ultraviolet part of the electromagnetic spectrum.
c. For the Sun with surface temperature T = 5800 K, so
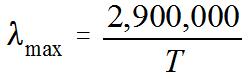 = 500 nm.
= 500 nm.
This is in the visible part of the electromagnetic spectrum.
d. For an interstellar cloud of gas, ices, and dust, T = 15 K, so
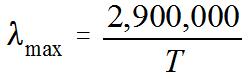 = 193,333 nm.
= 193,333 nm.
This is in the infrared part of the electromagnetic spectrum.
e. For the universe as a whole, T = 2.7 K, so
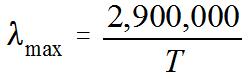 = 1,074,074 nm.
= 1,074,074 nm.
This is in the microwave part of the electromagnetic spectrum.
5. The energy of a photon is E = h v = hc/ λ so, if photon 1 has twice the wavelength of photon 2, then the energy of photon 1 is half the energy of photon 2.
6. List the following from longest to shortest wavelength: blue light, gamma rays, infrared, radio waves, red light, ultraviolet, x-rays: radio waves, infrared, red light, blue light, ultraviolet, x-rays, gamma rays.
List the following regions of the electromagnetic spectrum from smallest to largest photon energy: blue light, gamma rays, infrared, radio waves, red light, ultraviolet, x-rays:
radio waves, infrared, red light, blue light, ultraviolet, x-rays, gamma rays.
7. An atom is the smallest possible piece of a chemical element. It is made of three types of particles: protons, neutrons, and electrons. The tiny central nucleus of the atom contains the massive protons and neutrons. The less massive electrons orbit about the nucleus. The electric force holds the negatively charged electrons in orbit around the positively charged nucleus. Each element has its own characteristic spectrum because the electron energy levels (or orbits) are different for different elements. The energy of a photon that is absorbed or emitted by an atom must be equal to the difference in the energies of two of the atom= s energy levels.
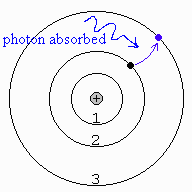 8. When a continuous spectrum is viewed through a cool gas, dark absorption lines appear in the continuous spectrum. This occurs because atoms in the cool gas absorb some of the photons at certain wavelengths from the continuous spectrum. In this case, electrons jump from lower/higher (circle one) to lower/higher (circle one) orbits in the atoms. The drawing at right shows an atom of hydrogen (one electron orbiting a single proton in the nucleus). Draw the electron and photon as the electron jumps from the second orbit to the third orbit when it absorbs the incoming photon. This particular process produces a dark absorption line in the spectrum of hydrogen at 656.3 nm, called the Hα absorption line.
8. When a continuous spectrum is viewed through a cool gas, dark absorption lines appear in the continuous spectrum. This occurs because atoms in the cool gas absorb some of the photons at certain wavelengths from the continuous spectrum. In this case, electrons jump from lower/higher (circle one) to lower/higher (circle one) orbits in the atoms. The drawing at right shows an atom of hydrogen (one electron orbiting a single proton in the nucleus). Draw the electron and photon as the electron jumps from the second orbit to the third orbit when it absorbs the incoming photon. This particular process produces a dark absorption line in the spectrum of hydrogen at 656.3 nm, called the Hα absorption line.
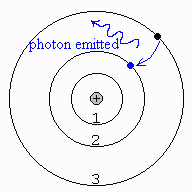 9. A hot, rarefied (less dense) gas produces bright emission lines. This occurs because atoms in the hot, rarefied gas emit photons at certain wavelengths. In this case, electrons fall from lower/higher (circle one) to lower/higher (circle one) orbits in the atoms. The drawing at right shows an atom of hydrogen. Draw the electron and photon as the electron falls from the third orbit down to the second orbit when it emits a photon. This particular process produces a bright emission line in the spectrum of hydrogen at 656.3 nm, called the Hα emission line.
9. A hot, rarefied (less dense) gas produces bright emission lines. This occurs because atoms in the hot, rarefied gas emit photons at certain wavelengths. In this case, electrons fall from lower/higher (circle one) to lower/higher (circle one) orbits in the atoms. The drawing at right shows an atom of hydrogen. Draw the electron and photon as the electron falls from the third orbit down to the second orbit when it emits a photon. This particular process produces a bright emission line in the spectrum of hydrogen at 656.3 nm, called the Hα emission line.
10. The speed of a star toward or away from us can be found from the Doppler shift of its spectral lines. The light from a star moving toward us has its wavelength compressed (shortened), so its light looks more blue. This is called a blueshift. The light from a star moving away from us has its wavelength stretched out (lengthened), so its light looks more red. This is called a redshift.
Return to Phys 1040 home page
Source: https://physics.weber.edu/carroll/phsx1040/HW/HW3Sp08_ans.htm
0 Response to "A Star Produces a Continuous Spectrum From Its"
Post a Comment